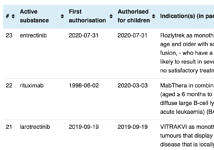
A continually updated list of centrally authorised products (updated 2026-01-14)
Ralf Herold's blog on progress of medical research and medicine development

A continually updated list of centrally authorised products (updated 2026-01-14)
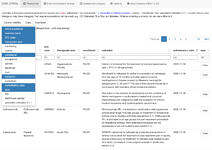
Interactive webapp about centrally authorised medicines and their assessments (EPARs), updated at least once a week
A list of tools such as biomarkers, simulation approaches, new endpoints, novel methods, commented by regulatory agencies, updated daily

A searchable webapp showing details of development plans (PIPs), updated at least once a week
 Many are relevant to be studied in children with cancer (updated 2026-01-24)
Many are relevant to be studied in children with cancer (updated 2026-01-24)Examples of medicines with development for children triggered by melanoma in adults (updated 2021-12-30)